Latest News
Aurrigo granted licence to provide ground handling services at East Midlands Airport
Aurrigo International plc, a leading international provider of autonomy software, fully autonomous vehicles and mobile robotics platforms, has been awarded a licence for the provision of Ground Handling Services at East Midlands Airport (EMA). The Coventry headquartered company has obtained this licence specifically to support its partners in the roll-out and implementation of its autonomous aviation […]
Manufacturing
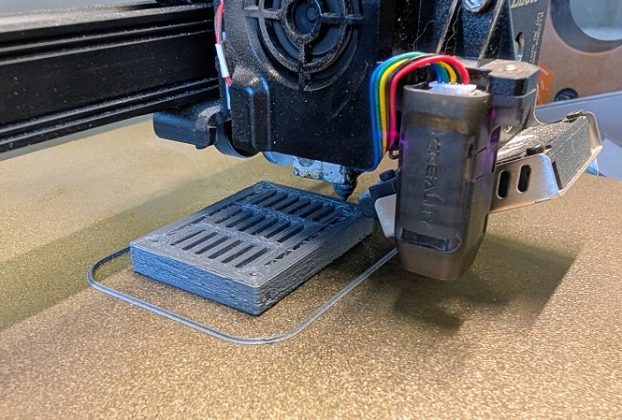
Greyhound Box Adopts 3D Printing to Improve Operations
Greyhound Box, a Leeds-based manufacturer of sustainable corrugated packaging, has integrated 3D printing technology to enhance its production processes and reduce costs. Known for its innovative approach and commitment to recyclable, FSC-certified materials, the company continues to drive efficiency and sustainability across the packaging industry. Greyhound Box has implemented 3D printing technology into its production […]
Events & Awards News
Event – Drive Technology Insight 2026: Advancing Precision Motion Engineering
Engineers developing motion-critical systems are invited to join maxon’s fourth Drive Technology Insight on 20 May at the British Motor Museum, Warwickshire. This one-day technical forum is for professionals working with high-performance DC drives, motion control, and advanced mechatronic architectures. The programme brings together engineering specialists to examine real application challenges and emerging motion technologies. […]
Company News
Your future in the Nuclear Industry
Postgraduate training that is personalised, flexible and taught by experts – that is what’s on offer from the Nuclear Technology Education Consortium (NTEC), whose unique course aims to create the UK’s next generation of nuclear experts, with the skills to secure a sustainable and safe nuclear industry. Seven educational organisations in the UK who work together as […]
Leading injection-moulding manufacturer increases capacity with £500k investment in modernisation
Leading injection-moulding manufacturer, Heyside Group, is set to increase its production capacity after investing £500k in a major shift from traditional manual processes to a fully modernised, automated factory. Heyside Group, a family-run firm which produces high-volume PVC injection-moulded products for the traffic management, utilities and infrastructure sectors from its Oldham manufacturing site, has secured […]
Product News
Rise to new dimensions with KNAPP
KNAPP is inviting IntraLogisteX visitors to rise to new dimensions of innovation, efficiency and sustainability in production and warehouse environments. The company will present its broad portfolio of automation and software solutions, many of which harness the power of robotics and AI to drive performance. Taking pride of place on stand 410 will be a display […]
Features & Interviews
International Women’s Day: Three Women in UK Manufacturing Share Their Story
On 8 March 2026, we celebrated International Women’s Day – shining a spotlight on the impact women have on our society and discovering how our sectors can do better in terms of championing women at work. Nowhere are these lessons more important than in the manufacturing industry, which proves to be challenging for women to […]
Latest Trends & Technology
Menlo Micro MM5627: First Fully Integrated, Highly Configurable 80 Gbps Differential DP3T Switch for Advanced AI Device Validation
Menlo Microsystems, Inc. (Menlo Micro), a leader in high-performance switches, has announced the MM5627, the first switch in its category to combine 80 Gbps performance with high configurability, integrated DP3T logic, and fully integrated on-chip components. Designed for high-speed test and validation of AI GPUs, CPUs, and advanced semiconductor ICs, the MM5627 enables customers to route, […]